将0.72g草酸亚铁(FeC2O4)放在一个可称量的敞口容器中高温焙烧,500~600℃时,容器中的固体质量保持0.4g不变。所得物质的化学式为( )
【分析】根据反应前后铁元素的质量不变,确定所得物质中铁元素和氧元素的质量比,进而确定其化学式。
【解答】解:0.72g草酸亚铁中含铁元素的质量为
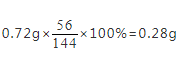
,而反应后固体质量为0.4g,不可能是铁单质,结合选项可知,应为铁的氧化物,铁元素和氧元素的质量比为0.28g:(0.4g﹣0.28g)=7:3,设铁的氧化物的化学式为FexOy,56x:16y=7:3,则x:y=2:3,故所得物质的化学式为Fe2O3。
故选:C。
【点评】本题难度不大,明确反应前后铁元素的质量不变、化学式的有关计算是正确解答本题的关键。


Dogs are the best pets of humans. They come in all sizes. Some dogs are as small as a football, and others are as tall as a desk. Dogs’ fur (毛) is different, too. It can be long and soft, or smooth and hard. People don’t mind the kind of fur, because all dogs are good to hug.


请在字典中查一下这个词。
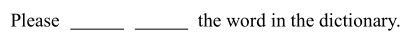
我喜欢甜食, 你知道的。

去年, 彼得在旅途中和许多人交了朋友。
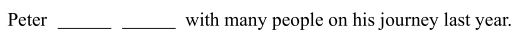
我叫托尼· 史密斯, 史密斯是我的姓。
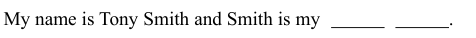
我们在课上回答问题时应该起立
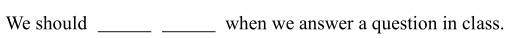

Tea and coffee are two of the most popular drinks in the world. However, people usually like one more than the other. These drinks also have very different uses.



People in the office usually sit for most of the day when they work. But this is bad for a person’s health. When people sit for too long every day, they don’t move very much. So it is easy to start getting fat. Sitting too long is also bad for their work because it makes them want to sleep. When this happens, people may make more mistakes in their work or spend more time finishing their work. Companies need workers to be healthy, so how do they fix the problem?

